A new guideline on Clostridium difficile infection (CDI) has been released in February 2018, covering changes in diagnosis and treatment recommendations.
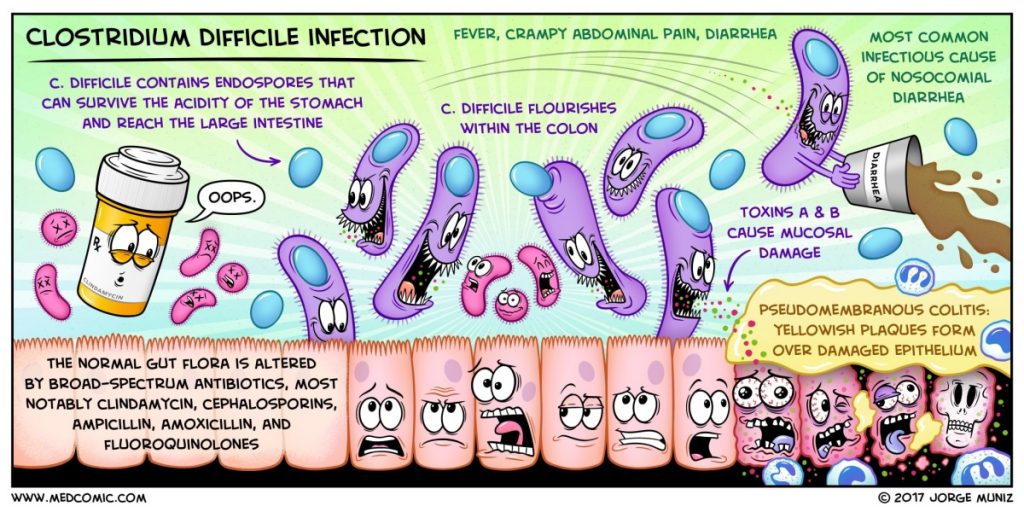
The highlights of the new guideline are:
- vancomycin or fidaxomicin are now first line therapy for mild/moderate CDI in adults, rather than the previously recommended metronidazole;
- in recurrent CDI, we can do either a a several-week tapered and pulsed course of vancomycin, or a 10-day course of fidaxomicin;
- in multiply recurrent CDI, fecal microbiota transplantation should be considered;
- regarding diagnosis, molecular tests (PCR) should be used in a two-step diagnostic algorithm (after GDH and toxin testing), in order to avoid misdiagnosing PCR positive patients with colonisation as having CDI
Endorsed by the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA), the new guideline is an update from the previous 2010 IDSA/SHEA CDI guideline.
The guideline is published in Clinical Infectious Diseases.
Foto: Medcomic; Reference: McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) [published online February 15, 2018]. Clin Infect Dis
